Proqr.com recives any estimated n/a unique visitors and n/a unique page views per day. Revenue gained from these much visits may be n/a per day from various advertising sources. The estimated worth of site is n/a.
- Website Age
n/a
- Alexa Rank no-data
- Country
 Netherlands
Netherlands
- IP Address
145.131.41.44
google ads
HTML SIZE INFORMATION
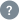
Text / Code Ratio
10.50 %
proqr.com has a website text/code ratio of 10.50 %. Search engine crawlers tend to not pick up pages with inadequate content.
IMPORTANT HTML TAGS AND COUNTS

H1
H4
| No |
Text |
| 1 |
How do rna therapies work? |
| 2 |
Leber’s congenital amaurosis |
| 3 |
Usher syndrome type 2 |
| 4 |
Retinitis pigmentosa (adrp) |
| 5 |
Axiomer® rna editing technology |
| 6 |
Meet us |
Text Styling

- STRONG0
- B0
- EM4
- I0
- U0
- CITE0
EM
| No |
Text |
| 1 |
Read more about sepofa****n for Leber's congenital amaurosis 10 and the clinical trial |
| 2 |
Read more about QR-421a and QR-411 for Usher syndrome type 2 |
| 3 |
Read more about QR-1123 for autosomal dominant retinitis pigmentosa |
| 4 |
Read more about Axiomer |
LINK ANALYSIS
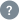
Total Link Count: 70
Internal Link Count
: 49 
| No |
Text |
Type |
| 1 |
- |
image |
| 2 |
Home |
text |
| 3 |
We are ProQR |
text |
| 4 |
About us |
text |
| 5 |
Vision 2023 |
text |
| 6 |
Leaders***p |
text |
| 7 |
Careers |
text |
| 8 |
Contact |
text |
| 9 |
Science & Pipeline |
text |
| 10 |
Research and development pipeline |
text |
| 11 |
Clinical Trials |
text |
| 12 |
QR-1123 Aurora phase 1/2 study for adRP |
text |
| 13 |
Sepofa****n ILLUMINATE phase 2/3 study for LCA10 |
text |
| 14 |
QR-421a STELLAR phase 1/2 study for Usher syndrome |
text |
| 15 |
Sepofa****n INSIGHT – phase 1b/2 study for LCA10 |
text |
| 16 |
Sepofa****n Study 001 phase 1/2 for LCA10 |
text |
| 17 |
Sepofa****n for Leber’s congenital amaurosis 10 |
text |
| 18 |
QR-421a for Usher syndrome type 2 |
text |
| 19 |
QR-1123 for autosomal dominant retinitis pigmentosa (adRP) |
text |
| 20 |
Axiomer RNA editing platform technology |
text |
| 21 |
Spin outs |
text |
| 22 |
Community |
text |
| 23 |
Patient and Medical Community Engagement |
text |
| 24 |
Eye Connect Newsletter |
text |
| 25 |
My Retina Tracker® genetic testing program |
text |
| 26 |
ProQR Expanded Access Policy |
text |
| 27 |
Stories |
text |
| 28 |
News & Publications |
text |
| 29 |
Posters & Publications |
text |
| 30 |
ProQR in the Media |
text |
| 31 |
Press kit |
text |
| 32 |
Details on the program |
text |
| 33 |
QR-1123 Aurora |
text |
| 34 |
QR-421a STELLAR |
text |
| 35 |
Sepofa****n ILLUMINATE |
text |
| 36 |
Read her story |
text |
| 37 |
Read their story |
text |
| 38 |
Read more |
text |
| 39 |
- |
image |
| 40 |
Read more about sepofa****n for Leber's congenital amaurosis 10 and the clinical trial |
text |
| 41 |
- |
image |
| 42 |
Read more about QR-421a and QR-411 for Usher syndrome type 2 |
text |
| 43 |
- |
image |
| 44 |
Read more about QR-1123 for autosomal dominant retinitis pigmentosa |
text |
| 45 |
- |
image |
| 46 |
Read more about Axiomer |
text |
| 47 |
Disclaimer |
text |
| 48 |
Privacy Policy |
text |
| 49 |
- |
empty |
External Link Count
: 21 
| No |
Text |
Type |
| 1 |
Wings Therapeutics |
text |
| 2 |
Amylon Therapeutics |
text |
| 3 |
Press Releases |
text |
| 4 |
Investors |
text |
| 5 |
Investor Home |
text |
| 6 |
Press Releases |
text |
| 7 |
Events and Presentations |
text |
| 8 |
Corporate Governance |
text |
| 9 |
Financial Information |
text |
| 10 |
Share Performance |
text |
| 11 |
Analysts |
text |
| 12 |
FAQ |
text |
| 13 |
Email Alerts |
text |
| 14 |
IR Contacts |
text |
| 15 |
Facebook |
text |
| 16 |
Twitter |
text |
| 17 |
LinkedIn |
text |
| 18 |
Play "How do ProQRs RNA therapies work" |
text |
| 19 |
ProQR investor conference call: Interim findings Phase 1/2 clinical trial of QR-421a |
text |
| 20 |
Annual meeting a***ociation for Research in Vision and Ophthalmology (ARVO) |
text |
| 21 |
ProQR Annual General Meeting of Shareholders |
text |
Nofollow Link Count
: 0
Title Link Count
: 0
WEBSITE SERVER INFORMATION
- Service Provider (ISP)
- Argeweb
- Hosted IP Address
- 145.131.41.44
- Hosted Country
 Netherlands
Netherlands- Host Region
- Provincie Utrecht , Utrecht
- Latitude and Longitude
- 52.0788 : 5.1347


 Netherlands
Netherlands


 External Link Count
: 21
External Link Count
: 21 